Australia's peak body for GPs has backed calls from federal Labor MP Julian Hill to ban the anti-acne and contraceptive pill which he says almost killed his daughter.

"Nearly a year ago a 64cm blood clot almost killed my healthy 20-year-old daughter, Elanor, after she was prescribed an old and dangerous drug, Diane-35," Hill said in a speech to parliament on Tuesday night.
Elanor complained of calf pain while the pair were on holiday in Sri Lanka in January and self-diagnosed as having deep vein thrombosis (DVT) before doctors at a hospital confirmed the clot in her leg.
"Since first talking publicly of our experience, I’ve been deluged with horror stories of life-threatening or fatal blood clots by families from across Australia.
"I have no hesitation in saying there are women in Australia who needlessly die or are harmed due to this drug."
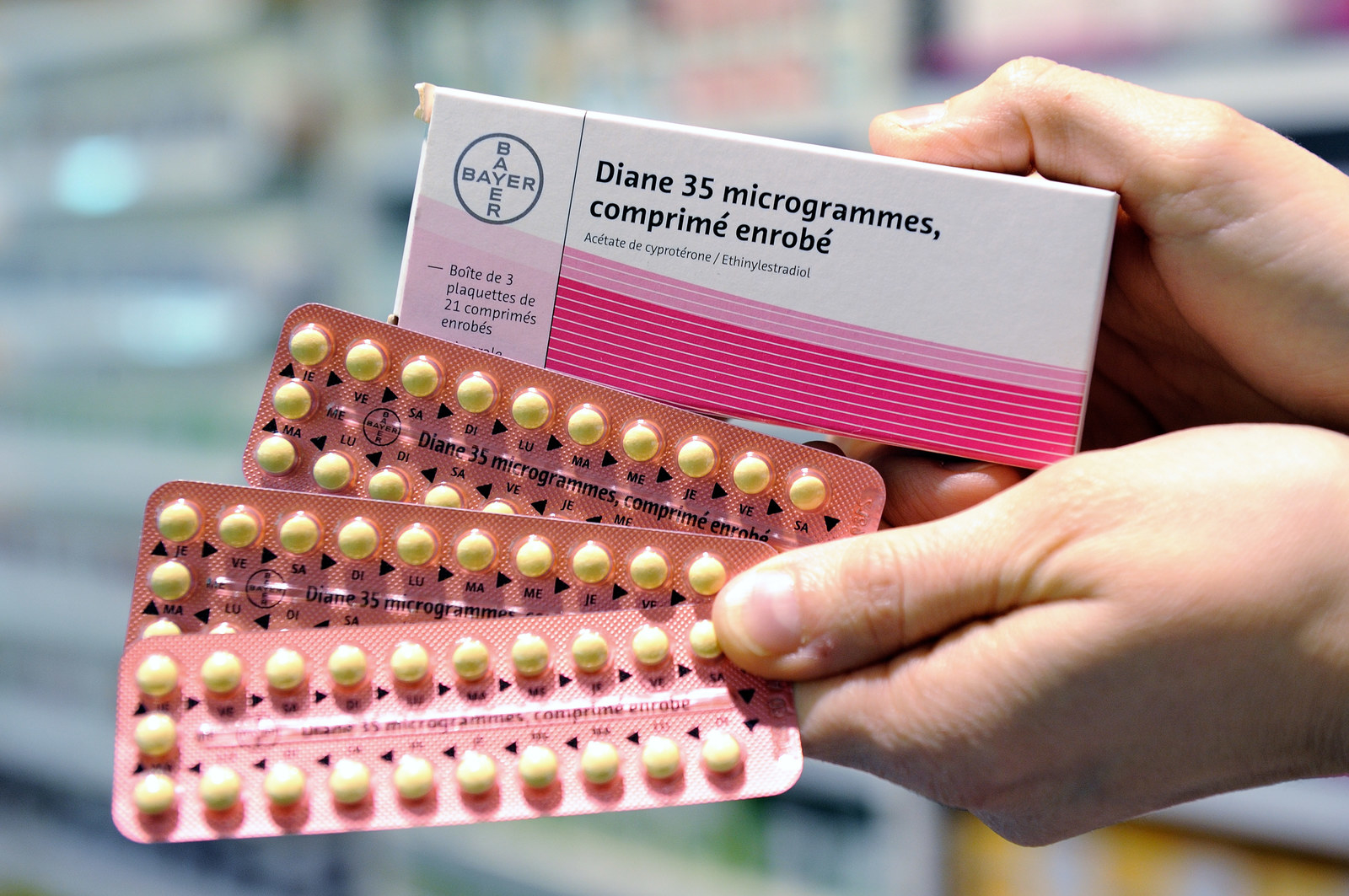
Diane-35 (also known as Diane 35 and Diane 35 ED) is also available under the trade names Brenda-35, Juliet-35, Estelle-35 and Laila-35. It's registered in Australia by the Therapeutic Goods Administration (TGA) for two issues: idiopathic hirsutism (excess hair growth in women) and acne.
It is not registered as a contraceptive but is but it is prescribed as an "off-label" (not on the Pharmaceutical Benefits Scheme) contraceptive.
Hill told the parliament that his daughter was never advised the medicine was an off-label prescription, warned that the risk of blood clots was higher than other contraceptives, or educated about warning signs.
Royal Australian College of General Practitioners (RACGP) president Bastian Seidel said the most concerning adverse effect caused by the drug was blood clots.

"In the case of Julian's daughter's DVT, if this blood clot had travelled up into the lung area it would cause a pulmonary embolism and it would have been potentially fatal," Seidel told BuzzFeed News.
"The side effects we are talking about are not a bit of an itch or a rash, we are talking about people dying."
In 2013, France's medical authorities confirmed 125 women had suffered "undesirable" and possibly life-threatening side-effects, and that four deaths in the past 25 years were linked to the drug.
Seidel echoed Hill's calls to ban the medication.
"Diane-35 is often prescribed to improve skin conditions and as a contraceptive, but there are various other options out there that are far better and safer for women to consider in the 21st century," he said.
"There must be a push to take this legacy medication off the market and to ensure there are better options out there for women."
Family Planning NSW medical director Dr Deborah Bateson said all oral contraceptives increased the risk of blood clots, but the risk was "very small" for most women.
"But pills like Diane-35 have a higher risk and should be reserved for women with severe acne, but even then those symptoms could be settled with a lower risk pill," Bateson told BuzzFeed News.
"There is anecdotal evidence these types of pills are over-utilised in Australia when there are lots of types of pills in the market with estrogen in them which are good for acne."
Bateson warned Australians on Diane-35 not to "stop taking their pill overnight" and instead talk to their doctor.
She also noted that long haul flights would increase the risk of clots for women on oral contraceptives.
Diane-35 is sold exclusively in Australia by Bayer.
"Bayer Australia Ltd is aware of the media coverage on its anti-acne medication, Diane 35 ED," a spokeswoman for the company told BuzzFeed News.
"Patient safety is of the utmost importance to Bayer and we are always saddened to hear of anyone experiencing an adverse event on any medication.
"Diane-35 has a favourable benefit-risk profile when used as directed, and this was confirmed by reviews conducted by both the European Medicines Authority and the TGA in 2013," she said.
"Bayer continuously reviews the safety profile of its products worldwide and collaborates with health and regulatory authorities, sharing all relevant information."
Further safety information related to venous thromboembolism was added to the Australian product information after the TGA review.
