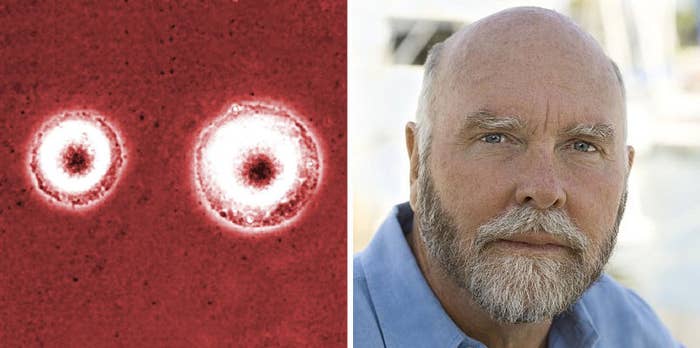
Scientists in California have created a cell with a "minimal" genome.
It has already revealed lots that we don't know about how life works. It could transform how we make things like medicines.
The researchers have taken the genes of a natural cell, modelled them on a computer, and stripped them out bit by bit until they just had the genes that were essential for life.
In a study published in Science, Dr Craig Venter and his team at Synthetic Genomics Inc show that they have redesigned the genome of a bacterium called Mycoplasma mycoides, stripping it back to just 473 different genes, down from about 900 in naturally occurring ones. For comparison, the human genome has about 20,000 to 25,000 genes.
It shows that it's possible to create entirely new, artificial genomes. And that means that scientists may be able to design bespoke bacteria which can churn out huge quantities of industrial chemicals, medicines and other substances we need.
The team has created artificial cells before, but this is the first time they've made their own, computer-designed genome for it.
In 2010, Venter and his colleagues sequenced the genome of M. mycoides. They then recreated that genome, and placed it in another bacterial cell, which grew as though it were M. mycoides.
However, the new study is different. They didn't just copy a ready-made genome – they redesigned one. "It's really exciting. The previous one was not really creating a life from scratch, it was more like a DNA transplant," Dr Ying Zhang, a researcher at Nottingham University's Synthetic Biology Research Centre who is not involved in the research, told BuzzFeed News. "This one can almost be called man-made life, rather than cheating a bit."
The team took the genome from their 2010 cell and divided it up into eight segments. Then they re-inserted each of the eight segments, one by one, into the genome, so they could test them, bring them back out again, and strip out genes until no more could be removed without the cell breaking down. The process took five years.

They've made the simplest cell known to humanity.
The original 2010 cell the team started with had 912 genes. One species of Mycoplasma has just 525 genes. This one is smaller still – the smallest genome of any known self-replicating cell.
But it's not the smallest possible genome that would make a reproducing cell. "It's a minimal genome, not the minimal genome," Venter told a press conference ahead of publication. "We had to produce something that grew fast enough to be a useful experimental model. It would probably have taken another five years if we hadn't insisted on rapid growth."
The process has thrown up a few surprises. For instance, there are a lot more "essential genes" than people realised, and scientists only know what two thirds of them do.
Venter said that, 20 years ago when they first started working on the synthetic genome project, it was thought that there would be between 250 and 300 essential genes. Instead, they couldn't get it below 473. And of those, 31% have unknown functions.
It also showed that what exactly counts as an "essential" gene is not straightforward. "When you knock out a gene from an organism, if the cell survives, you label it non-essential," said Venter. But that led to problems – lots of times, there were genes that seemed non-essential, but in fact were one of a pair. The cell could survive without either one, but not without both.
"It's like saying if you look at a 777, you could remove the engine from the right wing and the airplane can still fly and land, so it's non-essential," says Venter. "But you don't realise it's essential until you remove the second one."

As well as what it's taught scientists about the cell, it also has potentially major practical applications, for industry and medicine and agriculture.
"Right now, we can delete genes, insert genes, give a cell a new function," says Zhang. "But that's on a small scale.
"This is a first step to saying we can manipulate DNA on a much larger scale, hundreds of genes." That means, she says, creating bacteria that can churn out complex chemicals in huge quantities, because bacteria are much better at doing biochemistry than we are.
Dr Dan Gibson, one of the researchers on the team, told the press conference that the minimal genome "will be a very useful chassis for many industrial processes, for agriculture and medicine", because you can design bacterial cells which are maximally efficient for producing the chemicals you want.
"In theory," Venter said, "we could add gene sets and change this genome and essentially create any organism, eventually."
There's a long way to go from here to there, though.
"It's the start of a new era, but it won't happen overnight," said Gibson. That's partly because M. mycoides has been chosen because it's simple, not because it's chemically useful. "It's not an everyday lab strain that industry will be looking to use," said Zhang. "The next step will be to look to other bacteria like E. coli, to make it more streamlined, grow it faster in certain conditions to adapt to its industrial needs. You want it to be useful, to have some applications."
