1. Medicine spends YEARS (!!!) in testing and development before it reaches you.

2. That lengthy testing process is made up of multiple research studies called "clinical trials."

3. Clinical trials are really important — and for more reasons than you might think.

4. People participate in clinical studies for all sorts of different reasons.

5. Every clinical trial has its own Inclusion and Exclusion Criteria, aka rules about who can and cannot participate.

6. As with everything, the risks and benefits of joining a clinical trial are different for everyone.

7. The health, safety, and well-being of clinical trial participants are always taken extremely seriously...

8. …so seriously, in fact, that every clinical trial is overseen by doctors, an Ethics Review Board, and the FDA to ensure participants are being properly cared for and their rights are being protected.

9. Participants are always free to drop out of their clinical trial at any time, for any reason.

10. Clinical trials take place in a lot of different settings.

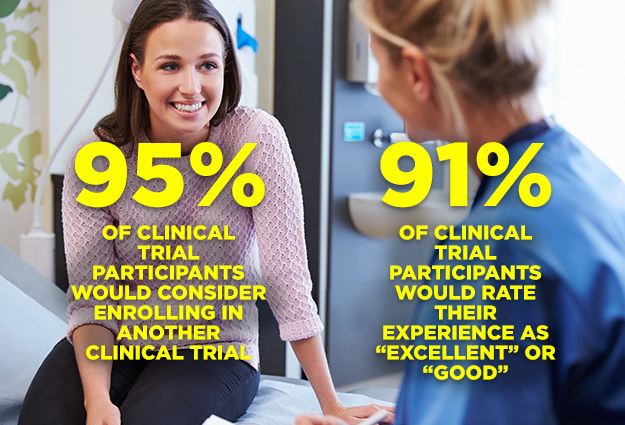
11. The majority of people who participate in clinical trials would recommend it!
12. Finding new and improved medicines is only possible because of people like you.

Wondering if a clinical trial is right for you? Lilly TrialGuide can help you find the answers you need.
All facts from Eli Lilly and Company
