A US federal agency has found that Orig3n, the startup behind a controversial plan to collect football fans’ DNA, doesn’t have the legal certification to sell genetic health tests, BuzzFeed News has learned.
Orig3n intended to give away genetic tests at a Baltimore Ravens game in September, but axed the promotion at the last minute in the face of questions from health officials. That led the Centers for Medicare and Medicaid Services (CMS) to examine the tests that Orig3n sells online for as much as $149.
At the time, the agency said it was working to determine whether Orig3n is subject to federal rules for laboratory testing of human samples. The company had privately argued that because it did not provide health information to individuals, it was exempt from those rules, known as the Clinical Laboratory Improvement Amendments (CLIA).
But in an Oct. 30 letter obtained by BuzzFeed News, a CMS official told Orig3n that it is, in fact, subject to CLIA rules because some of its tests — such as those purporting to assess customers’ athletic prowess and metabolism — measure people’s health. “To apply for CLIA certification, Orig3n must contact both the Massachusetts and California State Agencies immediately for guidance,” the letter said, referring to the states where Orig3n operates labs.
Orig3n’s various tests analyze 18 genes related to health, from “muscle power” to “sugar sensitivity” to “age-related metabolism,” wrote Karen Dyer, a director in CMS’s division of laboratory services, in her letter to Kate Blanchard, Orig3n’s chief operating officer. “It offers genetic testing that provides information for the assessment of health.”
Blanchard told BuzzFeed News that Orig3n’s products include 140 genes, and only 18 are subject to these federal rules. “We have been pursuing CLIA certification for some time,” Blanchard said in an emailed statement provided by a spokesperson. Until the company is certified, she said, Orig3n will keep selling tests that don't include those 18 genes.
“Going forward,” Blanchard said, “we will continue to work with CMS, California, Massachusetts and the other states to ensure that we are meeting all federal and state regulatory requirements regarding our products.”
The Boston startup was founded in 2014 and received $20 million in venture capital this summer. Its DNA tests have names like “Superhero” (“Are you faster than a speeding bullet? Do you have the mind of a super-genius?”) and “Fitcode” (“so you can get the info you need to fine-tune your routine and reach your goals faster”).
According to the CMS letter, Orig3n had described itself as a “research laboratory that does not provide patient specific results.” It claimed that its tests “are not offered to ‘patients’ but to ‘customers.’” And its terms of service says: “Orig3n’s genetic tests are not diagnostic tests and cannot predict your future health. We do not provide professional medical or clinical services or advice.”
But the agency did not find this argument convincing. CLIA regulations set standards for lab testing related to human health, such as a lab’s procedures for collecting, analyzing, and storing samples. CLIA certification also involves getting approval from the state where a laboratory is located.
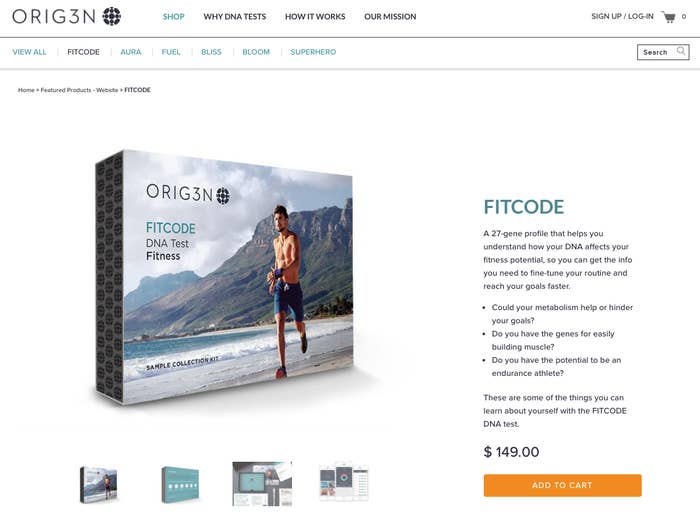
At the Sept. 17 Ravens game against the Cleveland Browns in Baltimore, Orig3n was planning to give away a test looking at four genetic variants.
The unusual promotion drew criticism from experts who told BuzzFeed News that the company made dubious scientific claims and could have compromised its customers’ privacy. For example, Orig3n had the right to sell or give their genetic data to a third party — like an advertiser or a drug company — as long as their name and other identifying information was removed.
What’s more, direct-to-consumer lab testing is generally prohibited in Maryland.
After receiving questions from BuzzFeed News before the promotion was canceled, Orig3n changed its privacy policy and terms of service to clarify how it handled customers’ genetic information. And CEO Robin Smith defended his tests. “We try to do our best to make sure these are based on real scientific studies that are out there and published in the world,” he said at the time.
According to the letter, Orig3n must provide the CMS with an update on its applications for CLIA certification by Nov. 13.
