
When Amber Freed first told doctors her baby boy wasn’t able to move his hands, they said that wasn’t possible.
Freed had given birth to twins in March 2017. While her baby girl, Riley, squirmed and babbled and crawled through the first year of her life, her fraternal twin, Maxwell, was different. He didn’t crawl or babble like Riley did. “I would fill out their baby books each month, and Riley had met all of these milestones. Maxwell didn’t reach one,” she said. Most alarmingly, however, Freed noticed that he never moved his hands.
She knew the news was going to be bad when they sent her to the “sad room” at the hospital, a featureless conference space filled with grim-faced doctors, to hear the diagnosis.
“You take your baby to the doctor and you say, ‘He can’t move his hands.’ And they look at you and they say, ‘Of course he can,’” said Freed.
“Then they look for themselves, and you can see from the look on their faces that they have never seen anything like this.”
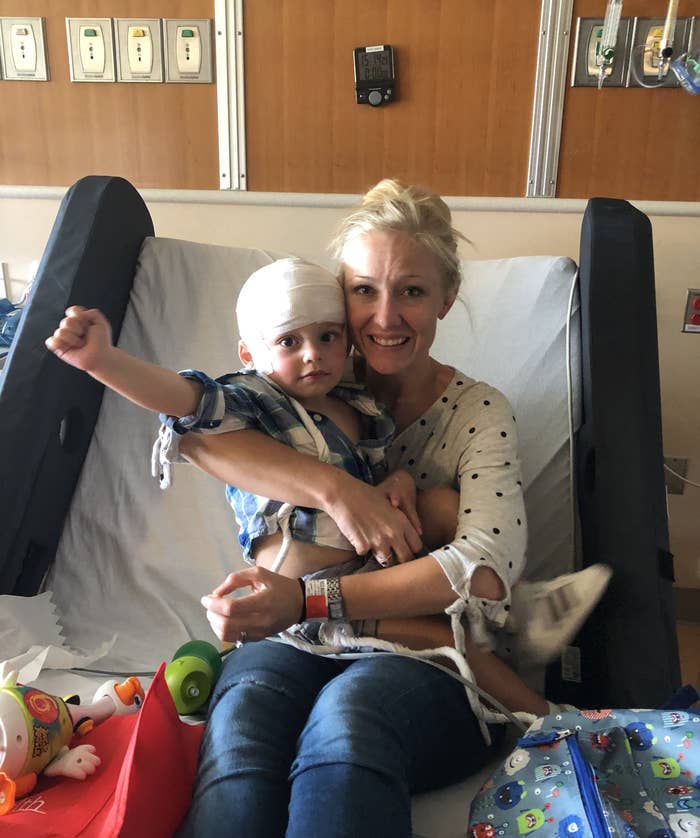
On June 14, 2018, at the Children's Hospital Colorado in Denver, Maxwell was diagnosed with a genetic disease called SLC6A1. The diagnosis explained why the infant hadn’t moved his hands or learned how to speak for the first year of his life, while Riley was thriving. But it didn’t explain much else: All the doctors who diagnosed Maxwell knew about the genetic disease came from a single five-page study published in 2014, the year of its discovery. It was too rare to even have a name, she was told, so the doctors just called it by the name of the affected gene: SLC6A1.
Now her 2½-year-old son is at the center of a multimillion-dollar race against time, one that’s come to include genetics researchers whom Freed personally recruited, paid for by $1 million that Freed and her husband, Mark, have raised themselves. At the center of their research will be specially crafted mutant mice that Freed paid scientists in China to genetically alter to have the same disease as Maxwell. The four mice are scheduled to arrive stateside next week, but Freed said she’s prepared to smuggle them into the US disguised as pets if there are any problems.
In total, Amber and Mark will need to raise as much as $7 million to test a genetic treatment for their child. And unless they can find — and fund — a cure, SLC6A1 will condemn Maxwell to severe epileptic seizures, most likely starting before he turns 3. The seizures may trigger developmental disabilities for a lifetime, often accompanied by aggressive behavior, hand flapping, and difficulty speaking.
And the Freeds will have to do it largely alone — there are only an estimated 100 other people diagnosed with SLC6A1 in the world. “This is the rarest of the rare diseases,” pediatric geneticist Austin Larson of the Children's Hospital Colorado told BuzzFeed News.
SLC6A1 is just one of thousands of untreatable rare diseases, and the perilous path it has set up for Freed, half science quarterback and half research fundraiser, is one that few parents can follow. “My dream is to create a playbook of how I did this for those that come after me,” said Freed. “I never want there to be another family that has suffered like this.”
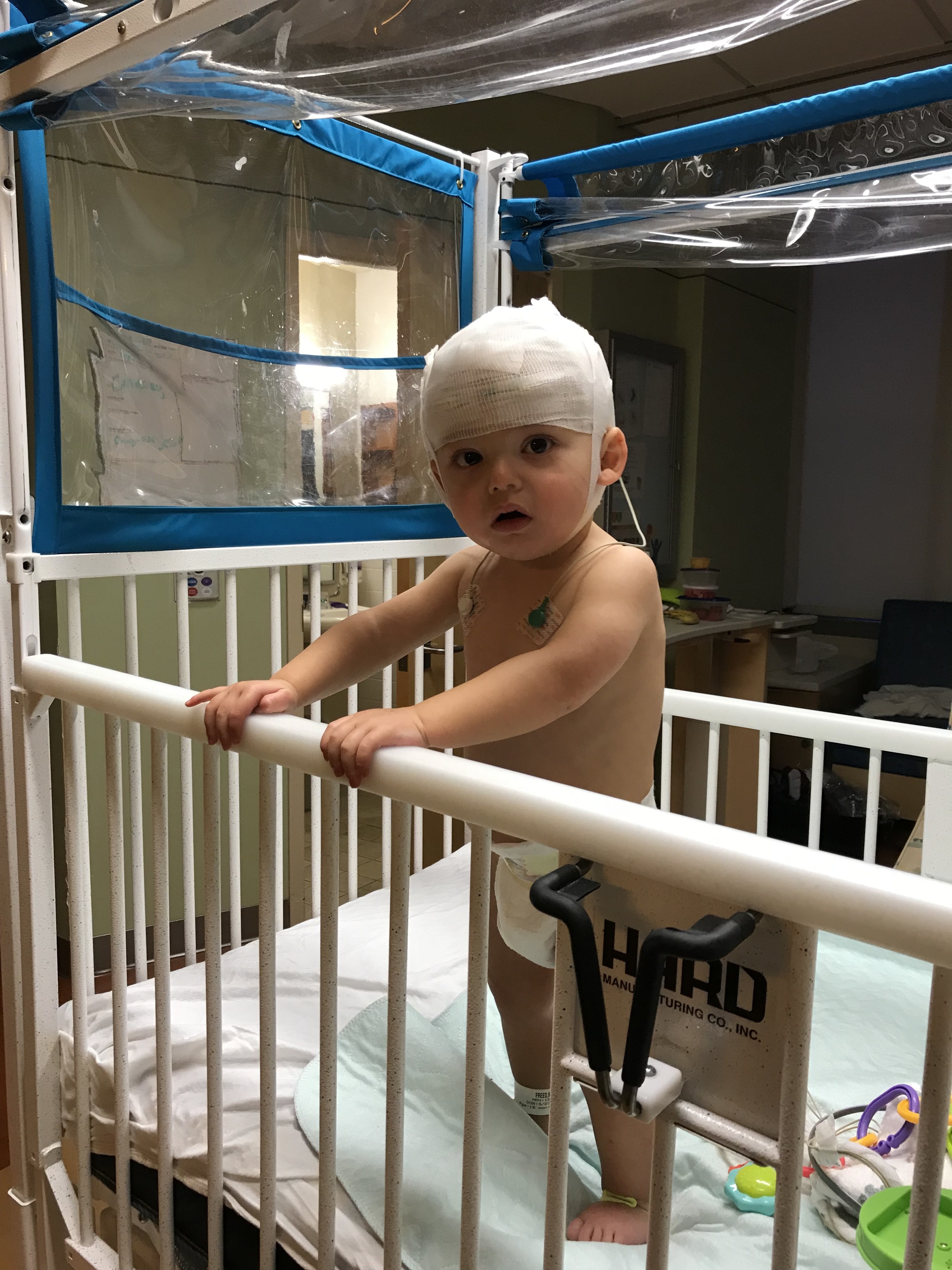
“You can think of SLC6A1 as a vacuum cleaner in the brain,” genetic counselor Katherine Helbig of the Children’s Hospital of Philadelphia, told BuzzFeed News. Helbig will speak at a conference on the gene at the American Epilepsy Society meeting in Baltimore on Dec. 5, an effort organized by Freed.
The protein made by the gene acts as a stop sign to message-carrying chemicals in the brain, halting them by vacuuming them up once they reach their destination brain cell, Helbig explained.
When one of the two copies of the SLC6A1 gene in every brain cell is damaged, like in Maxwell’s case, too little of its protein is available to perform its vacuuming duties, leading to miscommunication between cells, developmental disorders, autism-like symptoms, and, often, severe epileptic seizures.
Maxwell is about the age when epileptic seizures typically start in kids with the genetic disease, said Helbig, adding, “There probably are many more children out there who have it, but they just haven’t had the right test to find it.” At least 100 similar genetic defects cause similar kinds of epilepsy, afflicting about 1 in 2,000 kids, she said.
“I was the one who presented this diagnosis to Amber,” said Larson of the Children's Hospital Colorado. There was no medicine or diet or any other treatment for SLC6A1. It wasn’t an easy conversation. “Most of the time when we present a diagnosis for a genetic condition, there is not a specific treatment available.”
“At that moment, it was just vividly clear that the only option was for me to create our own miracle,” said Freed. “Nobody else was going to help.”
Half the battle with a rare genetic disease is getting researchers interested, said Helbig.
“At that moment, it was just vividly clear that the only option was for me to create our own miracle. Nobody else was going to help.”
So that is what Freed set out to do. She quit her job as a financial analyst and started making phone calls to scientists, calling 300 labs in the first three months. For those who didn’t respond, she sent them snacks via Uber Eats.
Her search, and a rapid-fire education on genetic diseases, led her to conclude the best hope for helping Maxwell was an experimental technique called gene therapy.
All the roads zeroed in on one scientist: Steven Gray of the University of Texas Southwestern Medical Center in Dallas. In 2018, a team headed by Gray reported the first human experiments of gene transfer by spinal injection, conducted in 5 to 10 children with mutations in a gene called GAN that causes swelling in brain cells.
The GAN gene transfer in that experiment, first tested in mice, attached a corrected version of the damaged gene to a harmless virus. Viruses reproduce by infecting cells and hijacking their DNA machinery to reproduce their own genes, making more viruses. The gene therapy virus in turn leaves behind a corrected gene in the DNA of cells they infect. Injected into the spinal cord, Gray’s virus can travel straight to the brain, leaving behind the corrected gene after the virus has run its course.
“I gave him my 30-second equity analyst pitch. I told him why Maxwell was a good patient, that we would raise $4 million to $7 million, and quarterback every step of the research,” she said. “And it worked. He agreed to make it a priority — if we could raise the money.”

Less than a month after meeting Gray, Freed contacted a lab at Tongji University in Shanghai that was also researching SLC6A1. The lab agreed to develop a mouse with Maxwell’s specific mutation for less than $50,000, using a gene modification technology called CRISPR that has revolutionized genetic engineering in the lab. “CRISPR mice are much more expensive in the US, and this lab had experience with the gene,” said Freed.
By July of this year, an experiment with a gene therapy virus that corrects SLC6A1 was tested on normal lab mice, which showed no sign of a toxic response, an encouraging sign. And by September, a line of CRISPR mice with Maxwell’s exact genetic mutation had been created at Tongji University.
“It is the literal mouse version of him,” said Freed. “Testing a therapy in this mouse is as close as science can get to testing in my son directly.”
To pay for all this, Maxwell’s family started fundraising last November and organized the first medical symposium on SLC6A1 in New Orleans that same month. They opened a GoFundMe account, which has raised $600,000, and held 35 fundraisers, which raised an additional $400,000 by October. In one charity competition, Larson from the Colorado Children’s Hospital, who diagnosed Maxwell, personally helped her raise $75,000.
“It is the literal mouse version of him. Testing a therapy in this mouse is as close as science can get to testing in my son directly.”
That money is helping to pay for the next step — getting the CRISPR mice to Gray’s lab to test the SLC6A1-correcting virus on them. But it’s not as simple as putting the mice in a box and shipping them by mail. The mice will be transferred through a lab at Vanderbilt University headed by Katty Kang, an expert on the neurotransmitter disrupted by Maxwell’s mutation.
“Amber is helping us to advance science, and everyone is making this a priority because of the young lives at stake — not just Maxwell, but other children this could help,” Kang told BuzzFeed News.
Once the four mice arrive, they will spend several weeks in quarantine, be tested to make sure they have Maxwell’s specific “point” mutation in the SLC6A1 gene, and breed with normal lab mice to produce generations of mixed-inheritance mice to serve as controls in future experiments. The mutant mice will be closely monitored before they head to UT Southwestern to make sure that they demonstrate the same problems and genetics as human patients with SLC6A1 and can therefore be used in any future clinical trials of gene therapy.
Right now at UT Southwestern, results from a safety test of the gene therapy virus — conducted by Gray’s lab on young, normal lab mice — is awaiting publication. If that works out, once the Chinese mice are sent over, they will also receive the gene-correcting virus. His team will see if their symptoms improve and to what extent their brain cells accept the corrected gene.
And then, Freed just needs another $5.5 million. Half a million dollars will go to test the virus in a second SLC6A1 animal model, likely a rat, as another safety step. Two million dollars will go toward creating more of the gene-correcting virus for a human safety study if that proves to be safe. And finally, if all that works out, $3 million will be needed to conduct the experiment on Maxwell and other children next year, following the path of the GAN clinical trial led by Gray.
“It’s a really horrible realization that the only thing standing in the way of a cure for your 2-year-old is money,” said Freed.
Freed acknowledges that she has only been able to pursue a cure for Maxwell because her family has the resources to do so — which she would never have had growing up in small towns in Texas, Montana, and Colorado in a poor family affected by alcoholism. “I grew up visiting my parents in rehab and knew what to say to put a family member on a 72-hour psychiatric hold by age 12,” she said. She dug herself out to build a career in finance, and hoped her kids would never have to experience the struggles she did growing up.
Even so, the fight hasn’t been easy on them — or on Maxwell’s sister, Riley.
Freed worries her daughter is growing up in doctors' waiting rooms, waiting on treatments for her brother to end. Maxwell’s disease has progressed, causing him to constantly clench his fingers, and sometimes pull his sister’s hair. His 3-year-old sister will gently remind him, “Soft hands, Maxie.”
Families like the Freeds are at the forefront of efforts to turn diagnoses of rare genetic ailments, which often used to be the stopping point for medicine, into treatments. A similar case saw the family of a 6-year-old girl, Mila Makovec, raise $3 million for gene therapy to cure her Batten disease, a deadly genetic brain disease that affects 2 to 4 of every 100,000 children born in the US.
In a New England Journal of Medicine editorial on that case published in October, FDA officials questioned how high the agency should set the safety bar for such treatments, meant for severe diseases affecting so few people. In these cases, parents are often collaborators in developing treatments, and might not want to stop efforts that come with high risks. “Even in rapidly progressing, fatal illnesses, precipitating severe complications or death is not acceptable, so what is the minimum assurance of safety that is needed?” wrote senior FDA officials Janet Woodcock and Peter Marks.
“This is way beyond what anyone expects of families.”
Finally, Woodcock and Marks wrote, “finding sustainable funding for such interventions may prove challenging, because the cost of production can be quite substantial, particularly for gene therapies.”
In our era of financial inequality, the specter of wealthy parents buying custom genetic treatments for their children’s ailments — while other parents desperately resort to GoFundMe accounts, or else do nothing — looms as a possibility.
“This is way beyond what anyone expects of families,” said Larson. The pathway has been opened up by the brave new world of improved genetic diagnoses, and the coming of age of rapid genetic engineering tools like CRISPR.
But only 20 years ago, an experimental gene therapy that relied on a “harmless” virus killed an 18-year-old volunteer, Jesse Gelsinger, in a research misconduct case that brought gene therapy to a standstill. Now more than 2,500 gene therapy clinical trials have been conducted, and more than 370 are underway. The human genome was not sequenced until 2000; today, mapping an entire human gene map costs around $700. In this new era, customized treatments for rare genetic diseases like Maxwell’s are suddenly possible.
“What I hope is that we are paving the way for other parents to help their children,” said Freed.
Families of children with rare genetic diseases are also working together to make treatments like the one Freed is spearheading possible, said Larson.
“They support each other and work together,” he said. The best example might be the families of children with cystic fibrosis, who — through the Cystic Fibrosis Foundation and the discovery of the gene responsible for the disease in 1989 — have pushed for the discovery of new drug treatments. In October, the FDA approved a “breakthrough” pharmaceutical that could treat 90% of cases.
“It is easier working with FDA on this kind of approach rather than starting from scratch,” Gray told BuzzFeed News by email. After all, he said, “it’s easier to follow a path that you’ve already walked down.”
Similarly, Freed hopes the SLC6A1 Connect advocacy group she started can lead to similar treatments for other children with genetic epilepsies caused by the gene.
“I don’t think any parent should be expected to single-handedly cure his or her child’s rare disease,” said Helbig. “Amber is a very tenacious and persistent person, and she will fight tooth and nail for her kids. But a lot of people don’t have the resources — and they shouldn’t have to.”
Helbig says that “cautious optimism” is appropriate on the chances of research yielding a genetic therapy for children like Maxwell. “For SLC6A1, it’s really too early to say whether this is going to work.”
But if it works, it might lead many more parents to get genetic tests for children that will reveal undiagnosed problems, she said. Many doctors discourage extensive genetic tests, thinking they won’t find anything helpful. In the absence of known treatments, insurers are also reluctant to pay for such tests, discouraging all but the most fortunate and resourceful parents. Even for them, there are no guarantees.
“The other tough reality is the possibility this treatment won’t be completed in time to help Maxwell,” said Freed. “I love him with every ounce of my being, and I want him to know that I did everything humanly possible to change his outcome.” ●
