On Wednesday, FDA chief Scott Gottlieb held a private meeting with patients, lawyers, and advocates to discuss Essure, the sterilization device that has been mired in controversy for years for reportedly causing serious complications in tens of thousands of women.
The popular device, a nonsurgical alternative to getting your tubes tied (tubal ligation), is a pair of metal and polyester coils inserted into the fallopian tubes. It induces the body to create scar tissue, which then blocks eggs from being fertilized and implanting in the uterus.
In 2016, after thousands of adverse event reports were submitted to the FDA — describing, for example, persistent pain, perforation of the uterus or fallopian tubes, abnormal bleeding, allergic reactions, and even unplanned pregnancies — the agency issued a black box warning for the device, the strongest warning label available. Because the science on Essure’s risks is unclear, the agency also demanded that Bayer launch a new safety study.
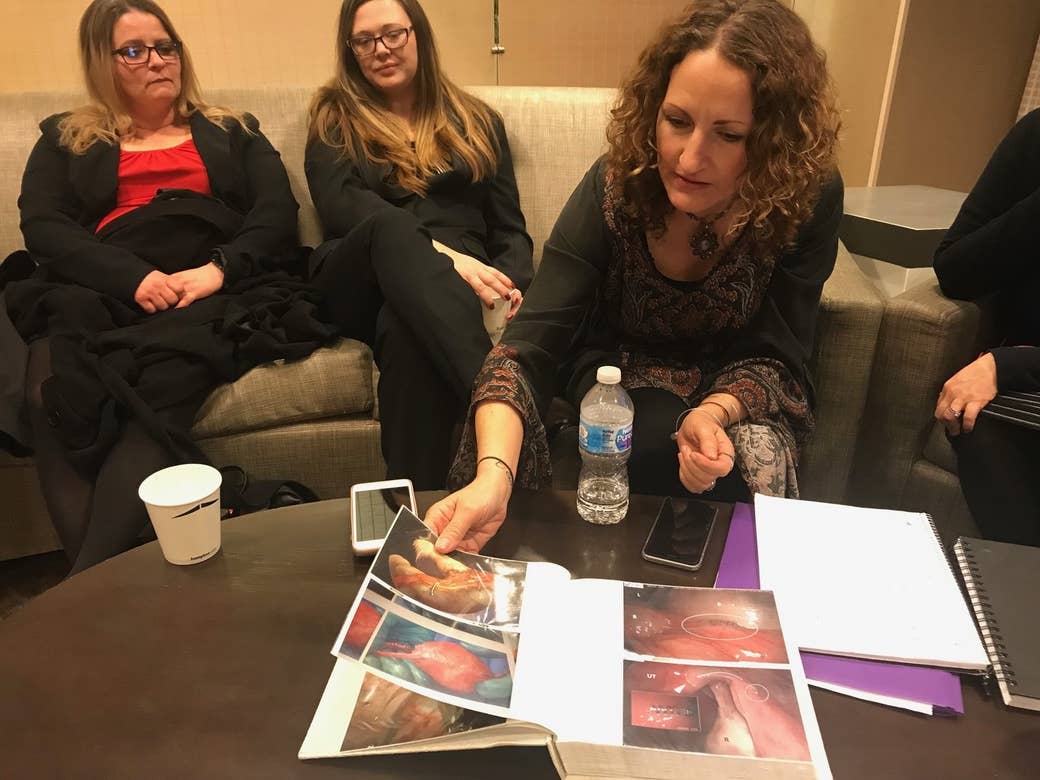
This week, Gottlieb met with some of the device’s loudest critics: five women who manage the Essure Problems Facebook group of more than 36,000 people. With the aim of convincing Gottlieb to temporarily pull the device from the US market, the group presented what they’ve seen over the last six years of advocacy and legal battles with the device’s manufacturer, Bayer — including graphic photos of broken coils implanted in uteruses, body rashes, stillbirths, dissolved fallopian tubes, and dental problems that they claim were caused by the device.
From slides on a laptop, the women also showed Gottlieb data collected from their Facebook group. In various surveys of 476 women who had joined their group since the black box warning was added, just 8 said their doctors had informed them about the warning, and 442 said they never heard of it (25 did not respond).
“It’s in the FDA’s lap now — are you going to let these women get implanted without informed consent?” Angie Firmalino, the founder of the group, told BuzzFeed News in a Washington, DC, hotel after the meeting with Gottlieb (press were not allowed).
“The group has now proven over the last two years that the guidance is not working,” she added. “It’s a failure to warn — it’s obvious.”

Firmalino started the group in 2011, frustrated with the cramping, bleeding, and joint problems caused after Essure’s metal coil was expelled from her fallopian tube and implanted, and later shattered, in her uterus. (Firmalino had her uterus removed in a procedure in 2014.) At first she just wanted to find if other people were experiencing similar complications. But the group quickly grew to include tens of thousands of women, many sharing horror stories that they believe were caused by the device, and bonding over their shared pain. (Several group members have “E-sisters” tattooed on their wrists.)
Firmalino and the other four women told BuzzFeed News that Gottlieb said Wednesday that he’s on their side, but needs to wait for Bayer to finish its safety study, which will include 2,800 women. As of October, the company had enrolled 136 patients, and the final report is expected by 2023.
Bayer spokesperson Courtney Mallon told BuzzFeed News that it has sold 750,000 devices worldwide. Last year, after the device was banned in Australia, Brazil, and suspended across Europe, the company took the device off the market in every country — except the US. (Bayer says the company pulled it off the market because there wasn't enough patient interest in permanent birth control.)
Meanwhile, in the US, the device continues to face legal challenges (including from many of the women in the Essure Problems group.) As of October, around 10,600 patients have sued Bayer for personal injuries.
Since Essure was approved in 2002, the FDA has received more than 26,000 complaints.
Since Essure was approved in 2002, the FDA has received more than 26,000 complaints, according to Madris Tomes, a former IT employee for the FDA who has since built her own system to easily search the agency’s public adverse event database. (The FDA said it is still counting up the total complaints from 2017, but as of Dec. 31, 2016, it had received 14,919.) Tomes also attended the meeting with Gottlieb.
FDA spokesperson Deborah Kotz told BuzzFeed News that the agency encourages doctors and women’s organizations to inform patients of Essure’s possible risks, and is "committed" to the Bayer study to "provide answers."
As for the survey data from Essure Problems, Bayer said it tells doctors to inform patients of all risks, and took aim at the motives of the group’s leaders.
“We would strongly encourage the public to exercise caution when using social media as a resource for safety information or meaningful data,” Mallon, Bayer's spokesperson, told BuzzFeed News by email.
“The public dialogue driven by these same individuals is part of a meritless campaign against Essure rather than a good faith effort to discuss and address patient safety,” Mallon added, citing a recent study of 105,000 women who underwent either Essure insertion or a tubal ligation.
That study, based on health insurance data from France, found that one to three years after insertion, Essure was associated with a higher risk of sterilization failure and repeat procedures, but did not increase the risk of medical complications, except in women with a history of allergies.
The company and the FDA both pointed to the study as evidence of the device’s safety. “The results appear to be consistent with previous assessments conducted by the FDA on Essure’s safety profile,” Kotz, the FDA spokesperson, said by email. But some independent researchers see it differently.
“For me the thing that stuck out was this idea that the use of [Essure] was associated with higher risk of gynecological complications,” said Aileen Gariepy, an assistant professor of obstetrics, gynecology, and reproductive sciences at Yale School of Medicine, who is conducting an independent study of Essure using health care information from patients in California.
“Having the procedure fail is a major risk," she said. "I have a different conclusion from what their data shows. This study is helpful but this is not the gold standard.”
The science is murky enough, the Essure Problems leaders say, that for now, the device should not be on the market. “Every single day we see new women join our group,” said Amanda Dykeman, another group administrator. “That’s been our driving force. To see those duplicated symptoms every single day for six years, you know it’s the device.”