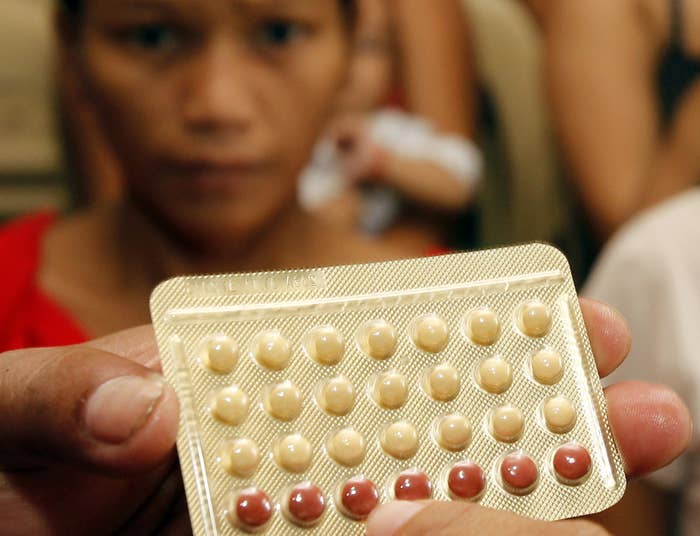
More than 100 women are suing a pharmaceutical company after they said a packaging error on birth control left them pregnant.
Qualitest Pharmaceuticals — a subsidiary of Endo International — recalled several brands of birth control in 2011 after finding that a packaging error misidentified placebo pills as active birth control. At the time, the Food and Drug Administration noted the error could put women at the risk of unintended pregnancy. Certain packs of brands including Cyclafem, Gildess, and Orsythia were included in the recall.
Now, 113 women have filed a lawsuit in a Pennsylvania court, saying the error did in fact lead to pregnancies, philly.com reported. The women filed their suit after a previous attempt to receive class action status in a federal court failed.
According to federal court documents, an attorney said he represented 117 women across 26 states who had used the birth control as directed by pharmaceutical company. Of those, 113 became pregnant, and 94 carried their babies to term.
The suit seeks millions of dollars to reimburse women for the money they spent on allegedly defective pills as well as damages for medical expenses, pain and suffering, injuries, and cost of raising the children caused by unwanted pregnancies.
The pharmaceutical companies earlier denied the allegations, adding that any women using the birth control pills were aware of risks. Any injuries were the result of unforeseeable events, or an act of God, and the company should not be held liable.
According to the court, a pharmacist in Iowa first returned three blister packs of Cyclafem 7/7/7 after seeing one of the packs had been turned upside down, hiding the expiration date. It was the only instance a defective package was known to have reached a consumer, the court said.
Following notification from the pharmacist, the company announced a recall, noting that certain packages were rotated 180 degrees. The error not only hid the expiration date, but also reversed the order of the rows of pills.
In the recall, 507,966 blister packs were returned, and 53 were found to have shown the reverse order.
Given that only a small number of the packs were wrongly labeled, a federal judge ruled that the lawsuit was not appropriate as a class action. Instead, cases should focus on individual women, their symptoms, and any proof available that the defective birth control pills resulted in pregnancy, the judge said.
